The Medical Technology (MedTech) market is experiencing significant growth globally, and Pakistan is no exception. As the demand for advanced medical devices and equipment increases, so does the complexity of navigating the import process. A comprehensive understanding of the regulatory landscape is crucial for ensuring the smooth and compliant importation of medical devices into Pakistan.
This guide aims to provide a roadmap for businesses involved in importing medical supplies, helping them navigate the regulatory framework and successfully access the Pakistani market.
Growing demand for devices in Pakistan
Pakistan is actively prioritizing the import of medical equipment, with an import value exceeding US$337 million (source: OEC) in medical instruments alone. This figure encompasses a wide array of equipment, ranging from basic tongue depressors to sophisticated in-vitro diagnostic (IVD) products and life-support systems like ventilators, incubators, and dialysis machines.
However, the country currently faces a significant shortage of medical devices due to import restrictions and other challenges. Successful importation of medical devices not only holds immense potential for businesses but also significantly benefits the medical industry in Pakistan.
By ensuring access to cutting-edge medical technology, the country can enhance patient care, improve healthcare outcomes, and maintain its upward trajectory in life expectancy, which currently stands at 67.94 years (source: macrotrends).
Understanding Pakistan's import regulations for medical devices
The import of medical devices into Pakistan is regulated by the Drug Regulatory Authority of Pakistan (DRAP). DRAP is the national regulatory body responsible for ensuring the safety, efficacy, and quality of all therapeutic goods, including pharmaceuticals and medical devices. It sets standards, issues licenses, and conducts inspections to ensure compliance.
Fortunately, the Government of Pakistan imposes no restrictions on foreign direct investment in healthcare services and allows medical equipment imports under its "Open General" regulations. This policy aims to encourage the import of medical devices to meet the growing needs of the healthcare sector. However, it's important to note that imports of radioactive equipment require additional approval from the Pakistan Nuclear Regulatory Authority (PNRA).
Key regulations and guidelines for importing medical devices
- Registration: All medical devices intended for import and sale in Pakistan must be registered with DRAP. This process involves submitting detailed technical documentation and evidence of compliance with international standards.
- Licensing: Importers of medical devices must obtain a valid import license from DRAP. This license authorizes the import of specific devices and ensures that the importer meets the necessary qualifications and facilities for handling such products.
- Labelling and Packaging Methods: All medical devices must comply with DRAP's labeling and packaging requirements, which include information on the product, manufacturer, and intended use.
- Good Manufacturing Practices (GMP): Manufacturers of medical devices must adhere to internationally recognized GMP standards to ensure the quality and safety of their products.
Classification of medical equipment in Pakistan
To streamline the registration and approval process, medical equipment in Pakistan are classified into four categories:
- Class A: Low-risk devices, such as bandages and tongue depressors, that require minimal regulatory scrutiny.
- Class B: Low-to-moderate risk devices, such as surgical instruments and diagnostic equipment, that are subject to more stringent registration requirements.
- Class C: Moderate-to-high-risk devices, such as implantable devices and life-support equipment, requiring extensive clinical evaluation and regulatory oversight.
- Class D: High-risk devices, such as heart valves and pacemakers, demanding the most rigorous regulatory scrutiny and post-market surveillance.
This classification system helps prioritize the evaluation and approval of essential medical equipment, ensuring that life-saving and critical devices are readily available to healthcare providers and patients across Pakistan.
Moreover, the development of special economic zones like the Hattar Industrial Estate, with its focus on pharmaceutical and medical device manufacturing, signals a commitment to strengthening domestic production capabilities and reducing reliance on imports in the long run.
Shipping and logistics considerations
Successful importation of medical equipment into Pakistan hinges not only on regulatory compliance but also on efficient shipping and logistics management. Careful consideration must be given to packing methods, handling, and documentation to ensure the safe and timely arrival of your pharmaceuticals and supplies.
1. Preparing for import
Before shipping medical supplies to Pakistan, importers must ensure that their products adhere to relevant international standards, such as ISO, CE marking, or FDA requirements, depending on the nature and intended use of the device. Compliance with these standards demonstrates the safety and quality of the products, facilitating the registration and import processes.
Moreover, importers need to adhere to Pakistan's product classification and compliance guidelines, as outlined by DRAP. This includes obtaining necessary licenses, import permits, and ensuring proper labeling and packaging methods of the medical devices.
2. Duties and taxes
While the Government of Pakistan encourages the import of medical devices, importers must be aware of the applicable duties and taxes liked to international shipping. Generally, medical devices are subject to customs duties ranging from 0% to 20%, depending on the specific product classification. Additionally, a standard Goods and Services Tax (GST) of 17% is levied on most imported goods, including medical devices.
To calculate the total cost of importing, including duties and taxes, importers need to consider the following:
- Cost, insurance, and freight (CIF) Value: This includes the cost of the goods, insurance, and freight charges up to the port of entry in Pakistan.
- Customs duty: The applicable customs duty rate based on the HS code of the product.
- GST: The standard GST rate of 17% applied to the sum of the CIF value and customs duty.
For instance, if you are importing a Class B medical device with a CIF (Cost, Insurance, and Freight) value of PKR 100,000 and the applicable customs duty rate is 5%, the customs duty would be calculated as follows:
Customs Duty = CIF Value x Duty Rate
Customs Duty = 100,000 x 0.05
Customs Duty = PKR 5,000
In addition to customs duty, a standard GST of 17% is applicable on most medical devices. Therefore, the total cost of importing the aforementioned device would be:
Total Cost = CIF Value + Customs Duty + GST
Total Cost = 100,000 + 5,000 + (105,000 x 0.17)
Total Cost = PKR 123,850
However, certain medical devices may qualify for duty exemption or relief under specific conditions. For example, donations of medical equipment to charitable organizations or public hospitals may be exempt from customs duties. Consult with a customs broker or refer to DRAP regulations to determine if your shipment qualifies for any exemptions or reliefs.
3. Shipping documentation
Accurate and complete documentation is paramount for smooth customs clearance and timely delivery. Ensure you have the following essential documents in order:
- Commercial invoice: A detailed invoice specifying the goods, their value, and other relevant transaction details.
- Packing list: An itemized list of the contents of each package, including quantity, weight, and description.
- Bill of lading/air waybill: The contract between the shipper and carrier, acting as a receipt for the goods and evidence of the contract of carriage.
- Import permit (if applicable): Required for certain categories of medical devices, such as Class C and D devices.
- Export permits and declarations: Depending on the country of origin, you may need export permits or declarations to comply with their export regulations.
Health certificates for importing medical devices, medical equipment, and pharmaceuticals into Pakistan include:
- Certificate of free sale (CFS): This certificate confirms that the medical device is legally marketed and sold in the country of origin.
- Certificate of analysis (COA): This document verifies the quality and composition of the product, ensuring it meets the required specifications.
- Other certificates: Depending on the specific product, additional certificates may be required, such as a Good Manufacturing Practice (GMP) certificate or a certificate of compliance with relevant international standards.
Licensing requirements for exporting medical devices will vary depending on the country of origin and the type of device being exported. However, it is generally recommended to obtain any necessary export licenses or permits from the relevant authorities in the exporting country to avoid any potential delays or complications at customs.
4. Packaging and labeling requirements
Proper packaging and labeling are critical for ensuring the safe and compliant arrival of medical supplies in Pakistan. Careful attention to these aspects protects delicate equipment and ensures smooth customs clearance.
Here are some best practices for packaging medical devices for import:
- Protective packaging: Use sturdy, high-quality packaging materials to safeguard your products from damage during transit.
- Internal cushioning: When packing fragile items like delicate surgical instruments or electronic components, use ample internal cushioning such as bubble wrap, foam inserts, or packing peanuts and similar methods to prevent movement and absorb shocks.
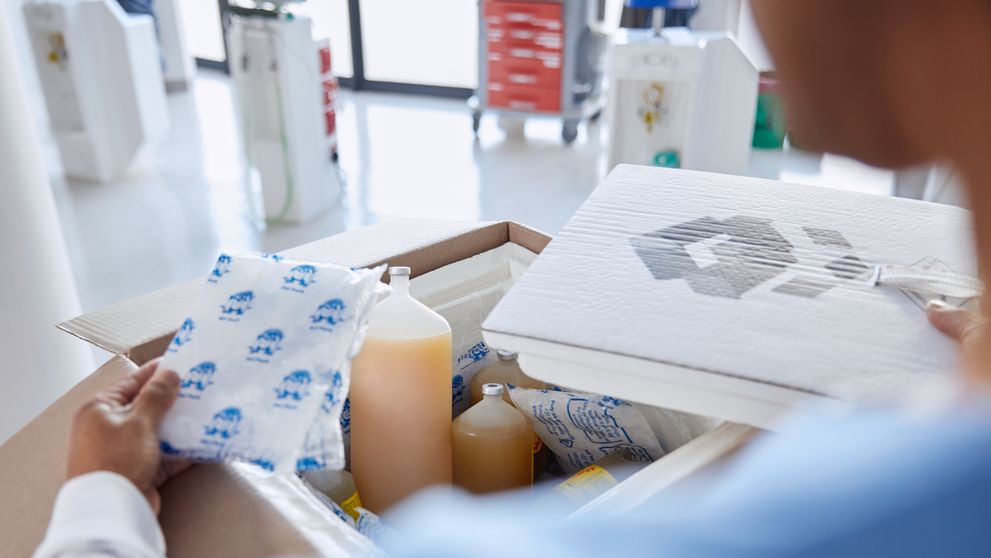
- Temperature control: If your medical devices are temperature-sensitive, ensure you utilize appropriate insulation and temperature-control measures, such as insulated boxes and gel packs. This will help maintain the required temperature range throughout the shipping journey.
- Clear labeling: Ensure all packages are clearly labeled with the consignee's address, product descriptions, HS codes, and any necessary handling instructions. Use both English and Urdu languages for labeling to facilitate customs clearance.
Explore DHL Express' medical express (WMX) service

For seamless and reliable transportation of medical devices and supplies to Pakistan, DHL Express offers its specialized Medical Express (WMX) service. This service is designed to meet the unique needs of the healthcare industry, ensuring the safe and timely delivery of temperature-sensitive products.
DHL Medical Express provides:
- Temperature-Controlled Solutions: We offer a range of temperature-controlled packaging and transport options to safeguard the integrity of your products.
- Real-Time Monitoring: Our advanced tracking technology allows you to monitor the location and temperature of your shipments in real time, ensuring complete visibility and control.
- Customs Expertise: Our experienced customs brokers can guide you through the complexities of import regulations and documentation, ensuring smooth and efficient customs clearance.
- Insurance and Risk Management: DHL offers comprehensive insurance options to protect your shipments against loss or damage during transit.
Partner with DHL Express to ensure your medical devices reach their destination safely and efficiently. Open a DHL business account today and leverage our Medical Express service for your import needs.